Whats New in Test Suite SP09
The new enhancements made to SAP Solution Manager’s Test Suite are strategic to regulated companies who employ different signature strategies for validating testing. These enhancements are beneficial for customers because of how it satisfies compliance standards. CoreALM has worked with many regulated companies where SAP Solution Manager’s digital signature process is fundamental to support their regulatory compliance. All of the signature enhancements will be a significant benefit for ensuring FDA title 21 CFR Part 11 compliance.
To see all of the enhancements across SAP Solution Manager SP09, visit
Explanation – What Changed? – Impact
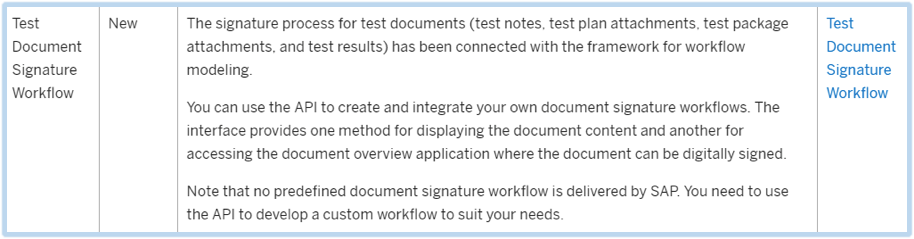
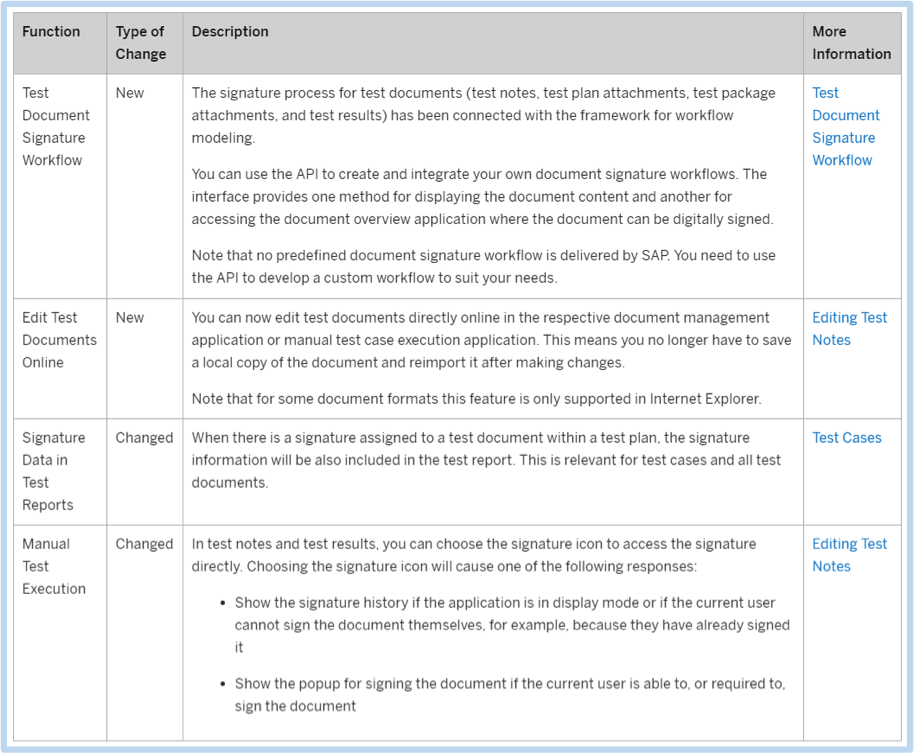
With this new functionality in SP09, users will be informed when their digital signature is required for test document, test plans, test plan or notes. In regards to a workflow, it can be used to prompt the user to sign the document.
SAP now allows you to use the API provided to create your own test document signature workflows. You may apply this to user’s signatures who are required to sign for test documents: test plans, test package attachments, test results, or test notes. With this new addition, the method for displaying the document content and accessing the document will aid in the document overview application.
For example, you could create a workflow so that when a particular status is set by a user, the new status will only take effect if the test manager confirms the new status with their digital signature. You could configure the workflow so that the test manager receives an email notification informing them that their signature is required. The email could contain one link to display the test document and another link to navigate directly to the relevant application where the test manager can provide their digital signature.
Explanation – What Changed? – Impact
The important thing to note is “This means you no longer have to save a local copy of the document and reimport it after making changes.” Obviously this removes a significant manual step and drives efficiency.
Explanation – What Changed? – Impact
For (FDA) regulated customers, being able to validate signatures via a report simplifies the compliance process, and again, drives efficiency. Resolving compliance issues as soon as possible is a benefit for everyone.
Explanation – What Changed? – Impact
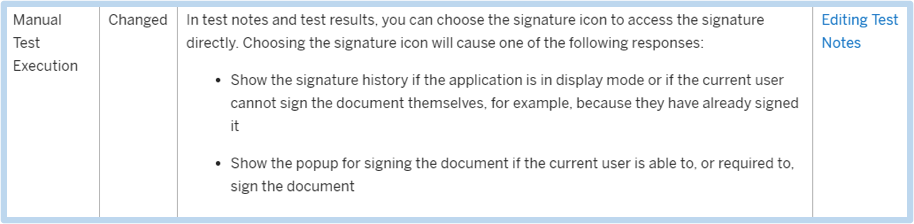
It is common that companies may have different signature requirements as based on their interpretation of various regulations. The benefit of this enhancement is the flexibility to support and automate different signature processes, which can all be executed in an efficient manner. The bottom line is that this new enhancement removes steps, thus simplifying the overall process.
The Benefits of Using Azure DevOps Connector for SAP Solution Manager Focused Build
Digital transformation is essential in today's business landscape, and SAP S/4HANA has been a game-changer…